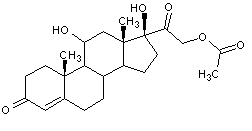
Structural formula
| Business number | 0134 |
|---|---|
| Molecular formula | C23H32O6 |
| Molecular weight | 404.5 |
| label |
Hydrocortisone-21-acetate, hydrocortisone acetate, hydrocortisone acetate, ifosfamide, Hydrocorticosterone acetate, Acetate Hydrogenated Adrenocortical Hormone, 21-Hydrocortisone acetate, Cortisol acetate, Hydrocortisone-21-acetate, Cortisol 21-acetate, 21-Acetoxy-4-pregnene-11b,17a-diol-3,20-dione, 17-α-Hydroxycorticosterone acetate, 17-Hydroxycorticosterone 21-acetate, 21-Acetoxy-4-pregnene-11β, 17α-diol-3,20-dione, 4-Pregnene-11β, 17α,21-triol-3,20-dione 21-acetate, 11β, hormone, antirheumatic drugs, anti-inflammatory drugs |
Numbering system
CAS number:50-03-3
MDL number:MFCD00037714
EINECS number:200-004-4
RTECS number:GM8960000
BRN number:2066841
PubChem number:24895620
Physical property data
1. Properties: White or off-white crystalline powder, odorless
2. Density (g/mL, 25/4℃): Undetermined
3. Relative Vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 222-225°C.
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 223
9. Specific rotation (º): [α]25D+166°
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Dissolution.��:Undetermined
Toxicological data
Acute toxicity: Rat subcutaneous LDL0: 250 mg/kg; mouse abdominal LD50: 2300 mg/kg; mouse subcutaneous LD50: 45050 ug/kg.
2. Subacute toxicity
Rat subcutaneous oral TDL0: 175 mg/kg/35D-I
3. Teratogenicity: mouse test : 60 mg/kg, DNA damage; 150 mg/kg, gene mutation.
4. Reproductive toxicity
Rat TDLo: 1 mg/kg, fetal growth retardation; Rat TDLo: 1500 mg/kg, fetal death;
Rat abdominal TDLo: 2 mg/kg, embryonic death; Rat subcutaneous TDLo: 350 mg/kg, fetal growth retardation
Mouse caliber TDLo: 125 mg/kg, fetal growth retardation; small Mouse subcutaneous TDLo: 250 mg/kg, fetal death;
Mouse subcutaneous TDLo: 75 mg/kg, fetal development retardation; Mouse subcutaneous TDLo: 84 mg/kg, fetal development retardation
Mouse muscle TDLo: 64mg/kg, little effect; mouse muscle TDLo: 320mg/kg; malformation
Mouse intestinal TDLo: 200 mg/kg;
Rabbit caliber TDLo: 13 mg/kg
Pig muscle TDLo: 100 mg/kg
Ecological data
Other harmful effects: This substance is harmful to the environment and special attention should be paid to water bodies. are hazardous items.
Molecular structure data
1. Molar refractive index: 103.75
2. Molar volume (cm3/mol): 318.1
3. Isotonic specific volume (90.2K ): 858.0
4. Surface tension (dyne/cm): 52.8
5. Polarizability (10-24cm3): 41.13
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 10
6. Topological molecular polar surface area (TPSA): 101
7. Number of heavy atoms: 29
8. Surface charge: 0
9. Complexity: 786
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 7
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14, Number of uncertain chemical bond stereocenters: 0
15, Number of covalent bond units: 1
Properties and stability
White or off-white crystalline powder, odorless. Mp218-221.5℃; Specific optical rotation [α] 25D+166° (dioxane); ethanol solution has maximum absorption at the wavelength of 240nm. Insoluble in water, slightly soluble in ethanol (1:230) and chloroform (1:150), hardly soluble in ether.
Storage method
Store at 0-6 ºC in a dark, sealed container.
Synthesis method
Using C3-C5 aliphatic ketone as solvent, adding a certain amount of acetate, it is synthesized from hydrocortisone, acetic acid and acetic anhydride.
Purpose
Mainly used to treat rheumatoid arthritis, rheumatic fever, gout, bronchial asthma, etc. Injections are used for tuberculous or purulent meningitis, tuberculous pleurisy, empyema, arthritis, tenosynovitis, tendon strain, sprain, nodular prurigo, lichen planus, etc. Eye drops are used for various types of ophthalmia. Creams are used for allergic or seborrheic dermatitis, pruritus, etc.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/102-4.jpgextended-reading:https://www.newtopchem.com/archives/44779extended-reading:https://www.newtopchem.com/archives/1093extended-reading:https://www.bdmaee.net/dmcha/extended-reading:https://www.bdmaee.net/nt-cat-t45l-catalyst-cas121-143-5-newtopchem/extended-reading:https://www.newtopchem.com/archives/44034extended-reading:https://www.newtopchem.com/archives/40012extended-reading:https://www.newtopchem.com/archives/42953extended-reading:https://www.bdmaee.net/niax-ef-700-tertiary-amine-catalyst-momentive/extended-reading:https://www.newtopchem.com/archives/1159


