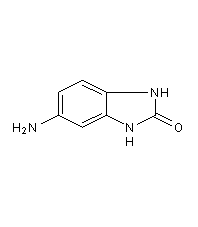
structural formula
| physical competition number | 028t |
|---|---|
| molecular formula | c7h7n3o |
| molecular weight | 149.15 |
| label |
5-amino-1,3-dihydro-2h-benzimidazol-2-one, 5-aminobenzimidazolones, 5-amino-1,3-dihydro-2h-benzimidazol-2-one |
numbering system
cas number:95-23-8
mdl number:mfcd00053555
einecs number:202-401-8
rtecs number:none
brn number:none
pubchem id:none
physical property data
1. character: light yellow or white crystal.
2. density (g/ml, 20℃): undetermined
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc): 239-244
5. boiling point (ºc, normal pressure): undetermined
6. boiling point (ºc, kpa): undetermined
7. refractive index: undetermined
8. flash point (ºc): undetermined
9. specific rotation (º): undetermined
p>
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (mmhg, ºc): undetermined
12. saturated vapor pressure (kpa, ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15. critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) distribution coefficient: undetermined
17. explosion upper limit (%, v /v): undetermined
18. lower explosion limit (%, v/v): undetermined
19. solubility: undetermined
toxicological data
none
ecological data
none
molecular structure data
1. molar refractive index: 40.26
2. molar volume (cm3/mol): 109.3
3. isotonic specific volume (90.2k ): 302.0
4. surface tension (dyne/cm): 58.1
5. polarizability (10-24cm3): 15.96
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 3
3. number of hydrogen bond acceptors: 2
4. number of rotatable chemical bonds: 0
5. number of tautomers: 3
6. topological molecule polar surface area 67.2
7. number of heavy atoms: 11
8. surface charge: 0
9. complexity: 183
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none
storage method
none
synthesis method
benzimidazolone is produced by solid-phase reaction of o-phenylenediamine and urea at 150-250°c to deaminate the hydrogen ring, and then undergoes nitration and reduction to obtain it.
purpose
mainly used to prepare yellow to orange organic pigments.
extended-reading:https://www.newtopchem.com/archives/44319extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/129-1.jpgextended-reading:https://www.bdmaee.net/niax-bdma-liquid-tertiary-amine-catalyst-/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-bl-13-niax-catalyst-a-133-niax-a-133.pdfextended-reading:https://www.morpholine.org/amine-catalyst-dabco-8154-catalyst-dabco-8154/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-rp208-high-efficiency-reaction-type-equilibrium-catalyst-reaction-type-equilibrium-catalyst.pdfextended-reading:https://www.morpholine.org/3-morpholinopropylamine/extended-reading:https://www.bdmaee.net/niax-a-305-gel-catalyst-/extended-reading:https://www.cyclohexylamine.net/lupragen-n105-pc-cat-nmm-dabco-nmm/extended-reading:https://www.newtopchem.com/archives/44293

