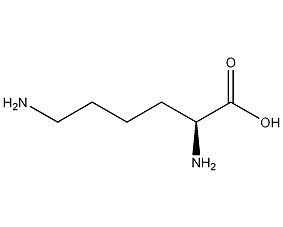
structural formula
| business number | 0181 |
|---|---|
| molecular formula | c6h14n2o2 |
| molecular weight | 146.19 |
| label |
(s)-2,6-diaminocaproic acid, (s)-2,6-diaminocaproic acid, (s)-(+)-lysine, amino acid drugs, intermediates, biochemical reagents |
numbering system
cas number:56-87-1
mdl number:mfcd00064433
einecs number:200-294-2
rtecs number:ol5540000
brn number:1722531
pubchem number:24896404
physical property data
1. properties: colorless needle-like crystals or crystalline powder. optically active.
2. density (g/ml, 25/4℃): 1.125
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc): it turns black when heated to 210℃ and decomposes at 224.5℃.
5. boiling point (ºc, normal pressure): undetermined
6. boiling point (ºc, 5.2kpa): undetermined
7. refractive index: undetermined
8. flash point (ºc): undetermined
9. specific rotation (º): α]d20 +14.6° (c=6.5, in water), [ α]d23+25.9° (c=2, in 6mol/l hydrochloric acid)
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure ( kpa, 25ºc): undetermined
12. saturated vapor pressure (kpa, 60ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15. critical pressure (kpa): undetermined
16. oil-water (octanol/water) partition coefficient pair value: undetermined
17. explosion upper limit (%, v/v): undetermined
18. explosion lower limit (%, v/v): undetermined
19. solubility: hygroscopic. very soluble in water, slightly soluble in ethanol, almost insoluble in ether.
toxicological data
1. reproductive toxicity: oral tdlo of female rats: 138mg/kg, conception takes 5-15 days; oral tdlo of female rats: 72450mg/kg, conception takes 10-20 days; oral tdlo of female rats: 90450mg/kg, conception occurs after 10-20 days; oral tdlo for female rats: 81 mg/kg, conception occurs after 10-20 days; intraperitoneal tdlo for female rats: 44 mg/kg, conception occurs after 5-15 days 2. mutagenicity: sister chromatid exchangetest system : human lymphocytes: <span lang=e body
ecological data
none yet
molecular structure data
1. molar refractive index: 38.43
2. molar volume (cm3/mol): 129.9
3. isotonic specific volume (90.2k ): 348.1
4. surface tension (dyne/cm): 51.5
5. polarizability (10-24cm3): 15.23
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 3
3. number of hydrogen bond acceptors: 4
4. number of rotatable chemical bonds: 5
5. number of tautomers: none
6. topological molecule polar surface area 89.3
7. number of heavy atoms: 10
8. surface charge: 0
9. complexity: 106
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 1
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. found in tobacco leaves.
storage method
this product should be sealed and stored dry and below 0℃.
synthesis method
1. l-lysine is generally supplied to the market as l-lysine hydrochloride [657-27-2]. free l-lysine is easily deliquescent and prone to yellowing and deterioration due to its free amino groups. , and has a pungent fishy smell, making it difficult to store for a long time. l-lysine hydrochloride is relatively stable, not easy to deliquesce, and easy to store. however, the demand for l-lysine in some uses is also increasing, such as peptide synthesis chemistry, biochemical research, and preparation of lysine derivatives. free l-lysine can be prepared from l-lysine hydrochloride.
2. tobacco: bu, 22; fc, 21; can be obtained by hydrolysis and refining of animal protein. it can also be synthesized from benzoyl piperidine.
purpose
for biochemical research. medium preparation.
extended-reading:https://www.bdmaee.net/niax-a-100-composite-amine-catalyst-/extended-reading:https://www.newtopchem.com/archives/219extended-reading:https://www.morpholine.org/category/morpholine/page/5395/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-dc5le-reaction-type-delayed-catalyst-reaction-type-catalyst.pdfextended-reading:https://www.bdmaee.net/bismuth-isooctanoate-cas67874-71-9-2-ethylhexanoic-acid-bismuth/extended-reading:https://www.newtopchem.com/archives/category/products/page/165extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/efficient-trimerization-catalyst-for-aliphatic-and-alicyclic-isocyanates.pdfextended-reading:https://www.bdmaee.net/fascat4202-catalyst-dibutyltin-dilaurate-arkema-pmc/extended-reading:https://www.bdmaee.net/kosmos-19-catalyst-cas121-73-6-degussa-ag/extended-reading:https://www.newtopchem.com/archives/39593

