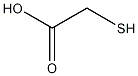
structural formula
| business number | 01f5 |
|---|---|
| molecular formula | c2h4o2s |
| molecular weight | 92.12 |
| label |
thiol acetic acid, thioglycolic acid, ethanethiol acid, hydrothioacetic acid, thioglycolic acid, thioglycolic acid, thioacetic acid, thioacetic acid, thioglycolic acid, thiol acetic acid, hydrothioacetic acid, thioglycolic acid, mercaptoacetic acid, 2-mercaptoacetate, 2-mercaptoacetic acid, tga, cold perm chemicals, polymer chain transfer agent, metal surface treatment agent, sensitive reagents for testing iron, molybdenum, aluminum, tin, etc. |
numbering system
cas number:68-11-1
mdl number:mfcd00004876
einecs number:200-677-4
rtecs number:ai5950000
brn number:506166
pubchem number:24900165
physical property data
1. properties: colorless transparent liquid with a strong unpleasant odor. [1]
2. melting point (℃): -16.5[2]
3. boiling point (℃): 120 (2.67kpa); 220[3]
4. relative density (water = 1): 1.33[4]
5. relative vapor density (air=1): 3.18[5]
6. saturated vapor pressure (kpa): 1.47 (104~06℃) [6]
7. critical pressure (mpa): 6.1[7]
8. octanol/water partition coefficient: 0.09 [8]
9. flash point (℃): 125[9]
10. ignition temperature (℃) ): 350[10]
11. explosion upper limit (%): no data yet
12. explosion lower limit (%): 5.9 [11]
13. solubility: miscible with water, miscible with ethanol and ether, and soluble in common solvents. [12]
toxicological data
1. acute toxicity[13] ld50: <50mg/kg (rat oral); 250mg/kg (mouse oral)
2. irritation no information available
ecological data
1. ecotoxicity no data yet
2. biodegradability[14] miti-i test, initial concentration 100ppm, sludge concentration 30ppm, 100% degradation after 4 weeks.
3. non-biodegradability[15] in the air, when the concentration of hydroxyl radicals is 5.00×105 pieces/cm3, the degradation half-life is 10h (theoretical).
molecular structure data
1. molar refractive index: 20.77
2. molar volume (cm3/mol): 70.2
3. isotonic specific volume (90.2k ): 185.3
4. surface tension (dyne/cm): 48.5
5. dielectric constant: none available
6. polarizability ( 10-24cm3): 8.23
7. single isotope mass: 91.993199 da
8. nominal mass: 92 da
9. average mass: 92.117 da
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): 0.1
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 1
5. number of tautomers: none
6. topological molecular polar surface area (tpsa): 38.3
7. number of heavy atoms: 5
8. surface charge: 0
9. complexity: 42.9
10. number of isotope atoms : 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the chemical bond configuration number of centers: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. rapidly oxidizes in the air, and the presence of a small amount of copper, iron, and manganese ions can accelerate the oxidation process. aqueous thioglycolic acid solutions with concentrations less than 70% by weight are stable when stored at room temperature. at high concentrations, a certain amount of various autoesterification products will be generated. when exposed to open fire or high heat, it will burn and release highly toxic hydrogen sulfide gas.
2. stability[16] stable
3. incompatible substances[17] strong oxidizing agent
4. conditions to avoid contact[18] heating
5.polymerization hazard[19] no polymerization
6. decomposition products[20] vulcanization object
storage method
storage precautions[21] store in a cool, ventilated warehouse. keep away from fire and heat sources. keep container tightly sealed. should be kept away from oxidizer, do not store together. equipped with the appropriate variety and quantity of fire equipment. the storage area should be equipped with emergency release equipment and suitable containment materials.
synthesis method
1. obtained from the reaction of inorganic or organic sulfur-containing compounds with sodium and potassium salts of monochloroacetic acid. for example: monochloroacetic acid reacts with sodium sulfide and sulfur to generate dithiodiacetic acid, which is then reduced with zinc and acid; or thiocarbamate reacts with monochloroacetic acid, and the product is obtained by hydrolysis; monochloroacetic acid and thiourea the reaction produces isothiouronoacetic acid, which is then converted to precipitate with barium hydroxide, and then acidified with sulfuric acid to produce a thioglycolic acid aqueous solution. after evaporation, a 60%-70% solution can be obtained with a yield of more than 70%.
![]()
2. the temperature does not exceed 25 ℃, add sodium carbonate solution to chloroacetic acid to a ph value of 7 to 8, and then add thiourea to react to generate urea acetate. the obtained thiourea acetate reacts with sodium hydroxide at a temperature of 78 to 80 ℃ for 2 hours. after the reaction is completed cool to 40°c, add concentrated hydrochloric acid until the ph value is 3, and then extract with ether. add activated carbon to the ether layer obtained by the extraction, stir thoroughly and filter. add zinc powder to the filtrate and distill under reduced pressure to obtain pure thioglycolic acid.
the process reaction formula is:
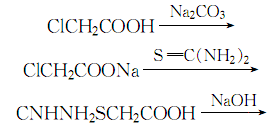
![]()
3. mix 16% chloroacetic acid aqueous solution with mix 15% potassium hydrosulfide aqueous solution, clch2cooh:khs=1:2 (molar ratio). heat, add a saturated solution of barium chloride with the same mass as chloroacetic acid, then add 25% concentrated ammonia water, stir evenly, let it stand, filter, add an equal volume of concentrated hydrochloric acid to the filtrate, then extract with ether, and distill the ether , finally, distill at 2000pa, collect the 104-106°c fraction, and obtain the finished product thioglycolic acid.
the reaction formula is:
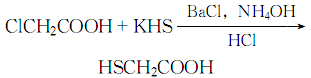
4. sodium hydrosulfide and chloroacetic acid are produced under the action of hydrogen sulfide and nitrogen.

5. chloroacetic acid, thio sodium sulfate and sodium hydroxide are synthesized, and then the finished product is obtained through acidolysis, extraction, and distillation.
purpose
1.tga has the reaction characteristics of both hydroxy acid and sulfhydryl group. the most important reaction is the reaction with disulfide. in particular, it reacts with cystine in hair under alkaline conditions, cutting the (-s-s-) bond of cystine to generate cysteine that is easy to curl. it is an intermediate for the production of pharmaceuticals such as caputolide, biotin, sulfur zinc acid, and sodium disulfide succinate. it is also an intermediate for the synthesis of cysteine, hormonal agents, industrial disinfectants, and an important raw material for sulfuric acid.
2. its ammonium salt and sodium salt are mainly used as curling agents, and its calcium salt can be used as depilatory agent, low-toxic or non-toxic stabilizer for polyvinyl chloride, initiator of polymerization reaction, accelerator and chain transfer agent, metal surface treatment agent. in addition, thioglycolic acid is a sensitive reagent for the determination of iron, molybdenum, aluminum, tin, etc.; it can also be used as a crystallization nucleating agent during polypropylene processing and molding, as well as a modifier for coatings, fibers, and a blanket speed conditioner. stabilizer raw materials for polyvinyl chloride and rubber, cold perming agents, and pharmaceutical intermediates.
3. used as a chromogen for the photometric determination of molybdenum, rhenium, iron, etc., and as a coordinating masking agent. its salts are also used in the preparation of cold perms and hair removers for curling hair.
4. hair removal agent: thioglycolate can quickly remove hair when applied to the skin under strong alkalinity. thioglycolic acid can be used for hair removal in cosmetology, pre-clinical surgery and animal experiments. the most commonly used is the calcium salt of thioglycolic acid, and the optimal ph value is 11.5 to 12. the minimum effective content is: 5% thioglycolic acid, 7% calcium salt.
5. adding a very small amount of thioglycolic acid to metal surface treatment agents can improve the cleaning effect of metal cleaning agents and impregnating solutions, and can inhibit corrosion of the metal surface being treated, so it is often used in precision machining of metals. of rust inhibitors. this effect is significant over a wide range of ph values. multi-component cleaning and anti-rust fluid containing thioglycolic acid can be used for cleaning automobile cooling systems with a ph value of 10.6. cetyl thioglycolic acid can polish or clean silver products without tarnishing the surface.
6. polyoxyethylene low-toxic or non-toxic stabilizer thioglycolic acid octylstannic acid, methyl tin ester, octyl ester, 2-ethylhexyl ester, methoxybutyl ester, ethoxyethyl ester they have low toxicity and non-toxicity. approved by the u.s. food and drug administration, the above-mentioned esters can be used as low-toxicity and non-toxic stabilizers. it can be used in the processing of pvc water pipes, water pumps and other products, food packaging, equipment pipelines in food processing plants, and can also be used in cosmetics and pharmaceutical factories. this type of stabilizer improves product transparency and thermal stability. . in addition, this product is a sensitive reagent for the determination of iron, molybdenum, lead, tin, etc. it can also be used as fiber modifier and blanket finishing agent.
7. used as a reagent and stabilizer for the determination of iron, in the manufacture of potions and perm lotions, etc. [22]
extended-reading:https://www.cyclohexylamine.net/delayed-catalyst-1028-delayed-catalyst/extended-reading:https://www.newtopchem.com/archives/category/products/page/109extended-reading:https://www.bdmaee.net/nt-cat-ba-25-catalyst-cas280-57-9-newtopchem/extended-reading:https://www.newtopchem.com/archives/category/products/page/22extended-reading:https://www.bdmaee.net/dibutyl-tin-bis-1-thioglycerol/extended-reading:https://www.morpholine.org/morpholine/extended-reading:https://www.bdmaee.net/n-formylmorpholine-cas4394-85-8-4-formylmorpholine/extended-reading:https://www.cyclohexylamine.net/lupragen-n206-tegoamin-bde-pc-cat-np90/extended-reading:https://www.newtopchem.com/archives/1766br>extended-reading:https://www.bdmaee.net/desmorepid-so-catalyst-cas112-96-9-rhine-chemistry/

