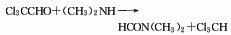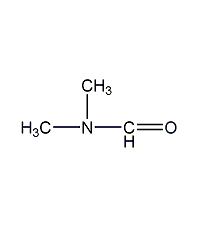
structural formula
| business number | 01f6 |
|---|---|
| molecular formula | c3h7no |
| molecular weight | 73 |
| label |
dimethylformamide, formamide, dimethylformamide, n-formamidedimethylamide;, dimethylformamide, dormyl dimethylamine, dmf, dmfa, n-formyldimethylamine, aliphatic nitrogenous compounds |
numbering system
cas number:68-12-2
mdl number:mfcd00003284
einecs number:200-679-5
rtecs number:lq2100000
brn number:605365
pubchem number:24893883
physical property data
1. properties: colorless, transparent or light yellow liquid with fishy smell. [1]
2. melting point (℃): -61[2]
3. boiling point (℃): 153[3]
4. relative density (water = 1): 0.95[4]
5. relative vapor density (air=1): 2.51[5]
6. saturated vapor pressure (kpa): 0.5 (25℃)[6]
7. heat of combustion (kj/mol): -1921[7]
8. critical temperature (℃): 374[8]
9. critical pressure (mpa): 4.48[9]
10. octanol/water partition coefficient: -0.87[10]
11. flash point (℃): 58 (oc) [11]
12. ignition temperature ( ℃): 445[12]
13. explosion upper limit (%): 15.2[13]
14. explosion lower limit (%): 2.2[14]
15. solubility: miscible with water and miscible in most organic solvents. [15]
16. refractive index (25ºc): 1.42817
17. viscosity (mpa·s, 25ºc): 0.802
18. specific rotation (º): 0.94
19. flash point (ºc): 445
20. heat of evaporation (kj/mol, 25ºc): 47.545
21. heat of evaporation (kj/mol, 100ºc): 43.585
22. heat of evaporation (kj/mol, b.p.): 38.368
23. heat of fusion (kj /mol): 16.165
24. heat of combustion (kj/mol): 1915.46
25. specific heat capacity (kj/(kg·k), 25ºc, constant pressure): 2.14
26. electrical conductivity (s/m): 6×10-8
27. thermal conductivity (w/(m·k), 20ºc ): 0.16579
toxicological data
1. acute toxicity[16]
ld50: 4000mg/kg (rat oral); 4720mg/kg (rabbit dermal )
lc50: 9400mg/m3 (mouse inhalation, 2h)
2. irritation [17] sup> rabbit eye: 100%, severe irritation (rinse with water)
3. subacute and chronic toxicity [18] when rats inhaled 2500mg/m3, 6 hours a day for 5 days, 8 to 10 out of 16 died. liver and lung damage could be seen at autopsy.
ecological data
1. ecotoxicity[19]
lc50: 1430mg/l (96h) (fathead minnow); 10000~13000mg/l (96h) (rainbow trout)
2. biodegradability no data available
3. non-biodegradability[20 ] in the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 22h (theoretical).
molecular structure data
1. molar refractive index: 19.85
2. molar volume (cm3/mol): 82.6
3. isotonic specific volume (90.2k ): 186
4. surface tension (dyne/cm): 25.7
5. dielectric constant (f/m): 36.7
6. couple polar distance (d): 3.86 (1d=3.33×10-30c·m)
7. polarizability (10-24cm 3): 7.87
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): -1
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 1
p>
4. number of rotatable chemical bonds: 0
5. topological molecular polar surface area (tpsa): 20.3
6. number of heavy atoms: 5
7. surface charge: 0
8. complexity: 33.9
9. number of isotope atoms: 0
10. determine the atomic stereocenter quantity: 0
11. number of uncertain atomic stereocenters: 0
12. number of determined chemical bond stereocenters: 0
13. uncertain chemical bonds number of stereocenters: 0
14. number of covalent bond units: 1
properties and stability
1. it is an aprotic polar solvent, has good dissolving ability for a variety of organic compounds and inorganic compounds, and has good chemical stability in the absence of alkali, acid, and water.
2. chemical properties: in the absence of acid, alkali and water, it is relatively stable even when heated to the boiling point. it decomposes into formic acid and dimethylamine salt under the action of acid, and decomposes into formate and dimethylamine under the action of alkali.
3. it is decomposed into dimethylamine and formaldehyde by the action of ultraviolet rays, and is decomposed into dimethylamine and carbon monoxide when heated to about 350°c. it forms a relatively stable equimolar adduct with hydrochloric acid, with a melting point of 40°c and a boiling point of 110°c. it can also form a crystalline adduct with so3, with a melting point of 138°c and a boiling point of 145°c. dmf-so3 can be used as a mild sulfonating agent and sulfating agent. adducts formed with pocl3, cocl2, socl2, etc. can introduce cho groups on aromatic rings with high electron density (vilsmeier reaction). p2o5 is insoluble in n,n-dimethylformamide at room temperature, but after forming a stable complex above 40°c, it can be dissolved at room temperature without precipitation. when heated in the presence of metallic sodium, a violent reaction occurs and hydrogen gas is released. it can also react violently with triethylaluminum at 0°c. can also react with grignard reagent. derivatives of diformamide are generated when reacting with acid chlorides and acid anhydrides.
4. it is of low toxicity. animal experiments have shown that continuous administration of large amounts of n,n-dimethylformamide can cause weight loss and hinder hematopoietic function. it has a strong irritating effect on the eyes, skin, and mucous membranes. its liquid or vapor can also cause liver disorders after being absorbed by the skin. inhaling high-concentration vapor can cause acute poisoning. the main symptoms are severe irritation, general spasm, painful constipation, nausea, and vomiting. in addition to skin and mucous membrane irritation, chronic poisoning may also cause nausea, vomiting, chest tightness, headache, general malaise, loss of appetite, stomach pain, constipation, hepatomegaly and changes in liver function, and urobilinogen and urobilinogen may also increase. when used, the average vapor concentration is required to be below 29.9mg/m3. poisoning symptoms (injury to the central nervous system) will occur when 59.8mg/m3. the oral toxicity ld50 of rats and mice is 3000~7000mg/kg. the olfactory threshold concentration is 0.14mg/m3, and tj 36-79 stipulates that the maximum allowable concentration in workshop air is 10mg/m3.
5. stability[21] stability
6. incompatible substances[22] strong oxidants, acid chlorides, chloroform, strong reducing agents, halogens, chlorinated hydrocarbons, concentrated sulfuric acid, fuming nitric acid
7. polymerization hazards[ 23] no aggregation
storage method
storage precautions[24] stored in a cool, ventilated warehouse. the storage temperature should not exceed 37℃. keep away from fire and heat sources. keep container tightly sealed. they should be stored separately from oxidants, reducing agents, halogens, etc., and avoid mixed storage. use explosion-proof lighting and ventilation facilities. it is prohibited to use mechanical equipment and tools that are prone to sparks. the storage area should be equipped with emergency release equipment and suitable containment materials.
synthesis method
since dimethylformamide was first synthesized by reacting formic acid and dimethylamine in 1899, processes for synthesizing dimethylformamide using different raw materials have been developed, such as dimethylamine-carbon monoxide method, formamide-dimethyl amine method, hydrocyanic acid-methanol method, acetonitrile-methanol method, methyl formate-dimethylamine method, trichloroacetaldehyde-dimethylamine method, etc. however, the current industrial production abroad is still dominated by the dimethylamine-carbon monoxide method.
1. methyl formate-dimethylamine method: esterification of formic acid and methanol generates methyl formate, and then reacts with dimethylamine in the gas phase to generate dimethylformamide, and then recovers methanol and unprocessed by distillation. after the reaction of methyl formate, the pressure was reduced.distillation to obtain finished products.
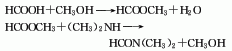
2. dimethylamine-carbon monoxide method: obtained from the direct reaction of dimethylamine and carbon monoxide under the action of sodium methoxide. reaction conditions are 1.5-2.5mpa and 110-150°c. the crude product is distilled to obtain the finished product.
![]()
3. made of carbon monoxide and methanol in methyl formate is obtained through oxo synthesis under high pressure and temperature of 80-100°c, and then reacts with dimethylamine to form dimethylformamide, and the finished product is obtained after distillation.
![]()
4. trichloroacetaldehyde method : obtained from the reaction of trichloroacetaldehyde and dimethylamine.
add chloroform and 0.52 parts of trichloroacetaldehyde into the reaction kettle, cool it to below 30°c, introduce gaseous dimethylamine, and at the same time, add 0.78 parts of trichloroacetaldehyde dropwise into the reaction kettle. , react. after the reaction, a distillation operation is performed. when the temperature at the top of the distillation tower is 58-64°c, the fraction is chloroform, and the fraction at 64-150°c is a mixture of chloroform and dimethylformamide. this mixture is distilled under reduced pressure. the crude dimethylformamide is obtained, and then the crude crystal is distilled to obtain the finished product.
![]() consumption quota (kg/t): dimethylamine (40%) 2372; trichloroacetaldehyde (95%) 2543.
consumption quota (kg/t): dimethylamine (40%) 2372; trichloroacetaldehyde (95%) 2543.
refining method: n,n-dimethylformamide often contains impurities such as water, ethanol, primary amines, and secondary amines, and can form hcon(ch3)2·2h2o with 2 molecules of water. to obtain high-purity products, a combination of desiccant and distillation can be used. first, add 1/10 volume of benzene and perform azeotropic distillation under normal pressure to remove water. then refine according to the following method:
① add anhydrous magnesium sulfate (25g/l) to dry, and distill under reduced pressure of 2~2.67kpa.
② add powdered barium oxide, stir, pour out the liquid, and distill under reduced pressure.
③ add alumina powder (50g/l, calcined at 500~600℃), mix and shake, and distill under reduced pressure (0.67~1.33kpa).
④ add triphenylsilyl chloride (5~10g/l), heat at 120~140℃ for 24 hours and then distill under reduced pressure (0.67kpa).
the conductivity of the product obtained by the above method: (1) (0.9~1.5)×10-7 s/m; (2) (0.4~1.0)×10-7 s/m; (3) (0.3~0.9)×10-7 s/m; (4) (0.2~0.5)×10-7 s/m.
5. use industrial product dimethylformamide as raw material and purify to obtain reagent dimethylformamide. if industrial products contain a small amount of water, it can be removed through 4a molecular sieves. if the moisture content is high, you can add an appropriate amount of granular potassium hydroxide, and leave it to stratify without shaking. after removing the water layer containing impurities such as formic acid, add reagent-grade benzene with 1/5 of the volume of dimethylformamide. distillation under normal pressure. when the gas phase temperature reaches 130°c, add an appropriate amount of phosphorus pentoxide to the residual liquid, cover it and shake for 3.5 hours. after letting it stand, filter out the solid, then dehydrate it with potassium hydroxide under nitrogen-filled conditions, and then distill under reduced pressure under the protection of dry nitrogen, and collect the middle fraction to obtain a high-purity product.
6. dimethylamine and carbon dioxide are synthesized under pressure under the catalysis of sodium methoxide or dimethylamine and methyl formate are reacted in the gas phase. it can also be obtained by the reaction of dimethylamine and trichloroacetaldehyde.
purpose
1. dimethylformamide is a good solvent for a variety of polymers such as polyethylene, polyvinyl chloride, polyacrylonitrile, polyamide, etc., and can be used for wet spinning of synthetic fibers such as polyacrylonitrile fiber. , synthesis of polyurethane; used in plastic film making; can also be used as a paint remover for removing paint; it can also dissolve some low-solubility pigments, giving the pigments the characteristics of dyes. dimethylformamide is used for the extraction of aromatic hydrocarbons and for the separation and recovery of butadiene from the c4 fraction and the separation and recovery of isoprene from the c5 fraction. it can also be used as an effective reagent for the separation of non-hydrocarbon components from paraffin. .
2. it has good selectivity for the solubility of isophthalic acid and terephthalic acid: the solubility of isophthalic acid in dimethylformamide is greater than that of terephthalic acid in dimethylformamide. the two can be separated by solvent extraction or partial crystallization from formic acid formamide. in the petrochemical industry, dimethylformamide can be used as a gas absorbent to separate and refine gases.
3. in organic reactions, dimethylformamide is not only widely used as a solvent for reactions, but also an important intermediate in organic synthesis. it can be used in the pesticide industry to produce pyrimidine.
4. reagents for titration of non-aqueous solutions, solvents for vinyl resin and acetylene, organic synthesis, photometry, gas chromatography stationary solution (maximum operating temperature 50°c, solvent is methanol), separation and analysis of c2-c5 hydrocarbons , and can separate normal and isobutylene] and cis and trans-butylene. pesticide residue analysis. organic synthesis. synthesis of peptides. for use in the photographic industry.
5. an excellent solvent and chemical raw material with extremely wide uses. it is an excellent solvent for a variety of polymers such as polyethylene, polyvinyl chloride, polyacrylonitrile, polyamide, etc. it can be used for wet spinning of synthetic fibers such as polyacrylonitrile fiber; synthesis of polyurethane; paint remover for paint removal; selective absorption of acetylene and separation and refining of butadiene; used as a solvent in the production of artificial leather; used in pesticides used to synthesize pesticides; in the pharmaceutical industry, it can be used to synthesize iodine, doxycycline, cortisone, vitamin b6, iodine glycosides, pyrantel, pyrantel, n-formylsarcolysin, and antineoplasmic acid. , methoxy mustard, benzene nitrogen mustard, ringhexyl nitrosourea, furfururacil, tournexamic acid, sefenmethasone, megestrol, cholevitamin, chlorpheniramine, etc.
6. used as a solvent and organic modifier in liquid chromatography, an extractant and developing agent for thin layer chromatography analysis, a solvent for vinyl resin and acetylene, and a solvent for titration of non-aqueous solvents. and used in organic synthesis.
7. it is mainly used as an industrial solvent. it is used in the pharmaceutical industry for the production of hormones and pesticides, and is also used in the manufacture of paramidine. [25]
extended-reading:https://www.newtopchem.com/archives/39799extended-reading:https://www.newtopchem.com/archives/44551extended-reading:https://www.newtopchem.com/archives/852extended-reading:https://www.newtopchem.com/archives/1049extended-reading:https://www.bdmaee.net/polyurethane-catalyst-a33-cas-280-57-9-dabco-33-lv/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/3-1.jpgextended-reading:https://www.newtopchem.com/archives/40226extended-reading:https://www.newtopchem.com/archives/category/products/page/88extended-reading:https://www.newtopchem.com/archives/44415extended-reading:https://www.bdmaee.net/niax-a-400-tertiary-amine-complex-catalyst-/