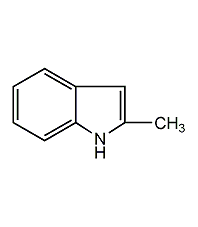
structural formula
| physical competition number | 028r |
|---|---|
| molecular formula | c9h9n |
| molecular weight | 131.17 |
| label |
2-methylindole, methylindole, 2-methyl-1h-indole, 2-methyl-1h-indole, α-methylindole |
numbering system
cas number:95-20-5
mdl number:mfcd00005616
einecs number:202-398-3
rtecs number:nm0345000
brn number:109781
pubchem number:24896980
physical property data
1. properties: white needle-like or flaky crystalline powder.
2. density (g/ml, 20℃): 1.07
3. relative vapor density (g/ml, air=1): undetermined
4. melting point (ºc): 61
5. boiling point (ºc, normal pressure): undetermined
6. boiling point (ºc, 0.67kpa): 272
7. refractive index: undetermined
8. flash point (ºc): 141
9. specific rotation (º): undetermined
10. autoignition point or ignition temperature (ºc): undetermined
11. vapor pressure (mmhg,ºc): undetermined
12. saturated vapor pressure (kpa,ºc ): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15. critical pressure (kpa): undetermined
16. log value of oil-water (octanol/water) distribution coefficient: undetermined
17. explosion upper limit (%, v/v): undetermined
18. lower explosion limit (%, v/v): undetermined
19. solubility: easily soluble in ethanol, ether, soluble in acetone, benzene, chloroform and sulfuric acid is slightly soluble in hot water and insoluble in cold water.
toxicological data
1. acute toxicity: mouse intraperitoneal ld50: >262mg/kg;
ecological data
slightly harmful to water.
molecular structure data
1. molar refractive index: 43.35
2. molar volume (cm3/mol): 106.2
3. isotonic specific volume (90.2k ): 308.3
4. surface tension (dyne/cm): 46.4
5. polarizability (10-24cm3): 17.18
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 1
3. number of hydrogen bond acceptors: 0
4. number of rotatable chemical bonds: 0
5. number of tautomers: none
6. topological molecule polar surface area 15.8
7. number of heavy atoms: 10
8. surface charge: 0
9. complexity: 122
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. the number of uncertain stereocenters of atoms: 0
13. the number of determined stereocenters of chemical bonds: 0
14. the number of uncertain stereocenters of chemical bonds: 0
15. number of covalent bond units: 1
properties and stability
can evaporate with water vapor. its ethanol, ether, benzene and chloroform solutions emit purple to blue-green fluorescence under daylight. decomposes when heated with concentrated hydrochloric acid. has a special smell. avoid contact with oxides and acids.
storage method
stored sealed in a cool, dry place. make sure the workspace has good ventilation. keep sealed. keep away from fire sources and store away from oxidants and acidic substances.
synthesis method
it is prepared from n-acetyl o-toluidine through the following steps. add n-acetyl o-toluidine to the mixture of anhydrous ether and sodium amide, heat to 240-260°c under the protection of nitrogen flow, and keep it for 10 minutes. the reaction will produce a large amount of gas. when the gas stops escaping, the reaction is completed and cooled. add ethanol and warm water, and heat to decompose the generated sodium derivative of 2-methylindole and excess sodium amide. after cooling, extract with diethyl ether. the extract is concentrated and then distilled, and the 119-126°c (0.4-0.53kpa) fraction is collected to obtain 2-methylindole with a yield of 80%-83%. this product can be purified by recrystallization from methanol.
purpose
it is used in organic synthesis and can be used as a fixative in the fragrance industry.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-nem-niax-nem-jeffcat-nem.pdfextended-reading:https://www.newtopchem.com/archives/44821extended-reading:https://www.newtopchem.com/archives/1859extended-reading:https://www.bdmaee.net/fascat9201-catalyst-dibutyl-tin-oxide-fascat9201/extended-reading:https://www.cyclohexylamine.net/catalyst-dabco-8154-acid-blocked-tertiary-amine-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/polyurethane-catalyst-a33-cas280-57-9-foaming-catalyst.pdfextended-reading:https://www.newtopchem.com/archives/39820extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-xd-102–amine-catalyst-amine-catalyst.pdfextended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/65.jpgextended-reading:https://www.bdmaee.net/octyl-tin-mercaptide/

